MORE ON EFFICACY
ZILXI was evaluated in a large population in 2 identical phase 3 trials and a 40-week open-label extension study12,13
1522 TOTAL PATIENTS*
18+ YEARS OF AGE WITH INFLAMMATORY LESIONS OF ROSACEA
TWO 12-WEEK TRIALS: RANDOMIZED, DOUBLE-BLIND, VEHICLE-CONTROLLED
40-WEEK OPEN-LABEL EXTENSION, SAFETY STUDY13†
Key inclusion/exclusion criteria12
Co-primary efficacy endpoints12
Safety evaluations12
*Trial 1 (2:1), N=751; Trial 2 (2:1), N=771.
†Subjects were eligible to enter the open-label extension study upon successful completion of either double-blind study (Trial 1 or Trial 2).
IGA = Investigator’s Global Assessment.
Reduction of inflammatory lesions occurred as early as week 4
‡ANCOVA, intent-to-treat.
ABSOLUTE REDUCTION OF INFLAMMATORY LESION COUNT


Patients receiving ZILXI demonstrated improvement in IGA success at week 12
IGA Treatment Success at Week 12


§IGA treatment success was defined as dichotomized (yes/no) IGA score of 0 or 1 and at least a 2-grade improvement from baseline at Week 12.
AT WEEK 12, THE PROPORTION OF SUBJECTS ACHIEVING IGA TREATMENT SUCCESS WITH ZILXI WAS SIGNIFICANTLY HIGHER VS VEHICLE
Post-hoc assessment of ZILXI across IGA disease severities
A post-hoc assessment pooled the co-primary efficacy endpoint data from Trial 1 and Trial 2 for analysis both in totality and for the predefined subgroup of baseline disease severity (moderate, IGA=3; or severe, IGA=4)14
IGA = Investigator's Global Assessment.
Integrated efficacy population (multiple imputation).
#Observed cases.
Effective Across Moderate to Severe IGA Disease Severities
40-week extension safety study
The multicenter, open-label extension study, which included 504 patients, evaluated the long-term safety, tolerability, and efficacy of ZILXI. Patients were eligible to enter the open-label extension study upon successful completion of either double-blind study (Trial 1 or Trial 2)13
| Number of subjects remaining in the study | ||
|---|---|---|
| Week | ZILXI 1.5% | MST-enabled vehicle ->ZILXI 1.5% |
| 0 | 332 | 172 |
| 4 | 332 | 172 |
| 8 | 331 | 172 |
| 12 | 332 | 172 |
| 16 | 326 | 168 |
| 22 | 321 | 156 |
| 28 | 310 | 150 |
| 34 | 298 | 146 |
| 40 | 286 | 138 |
| 46 | 279 | 136 |
| 52 | 272 | 129 |
Change from baseline is calculated as the value at baseline minus the post-baseline value.
IGA TREATMENT SUCCESS AT WEEK 12 AND THROUGH OPEN-LABEL EXTENSION STUDY


IGA = Investigator’s Global Assessment.
¶Week 12 of the double-blind study serves as the baseline for the open-label extension study.
PATIENTS SAW CONTINUED REDUCTION OF INFLAMMATORY LESION COUNT AND IMPROVEMENT IN IGA SCORES THROUGH WEEK 5213
ZILXI—see the difference of first-line power
IGA SCORE=4
IGA SCORE=1
BASELINE
WEEK 12
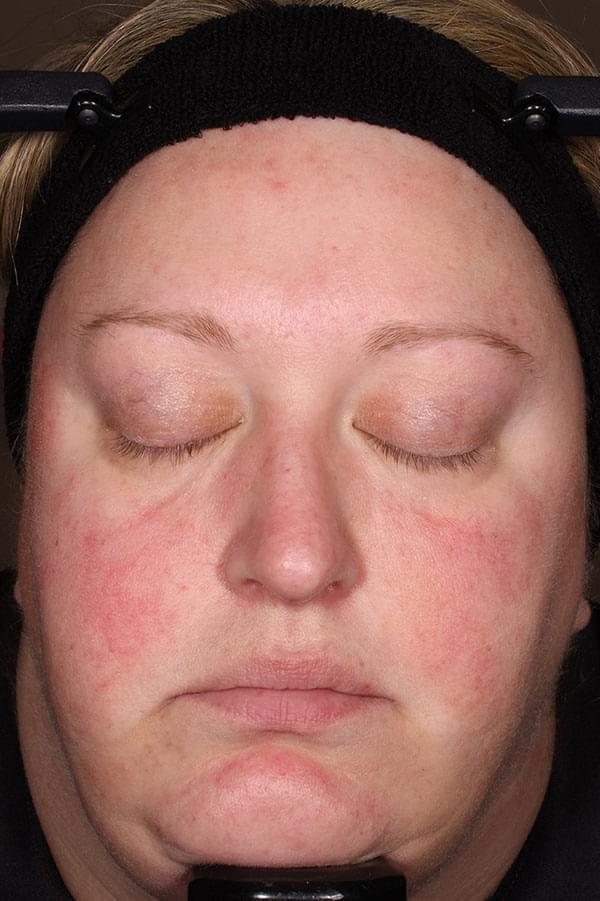
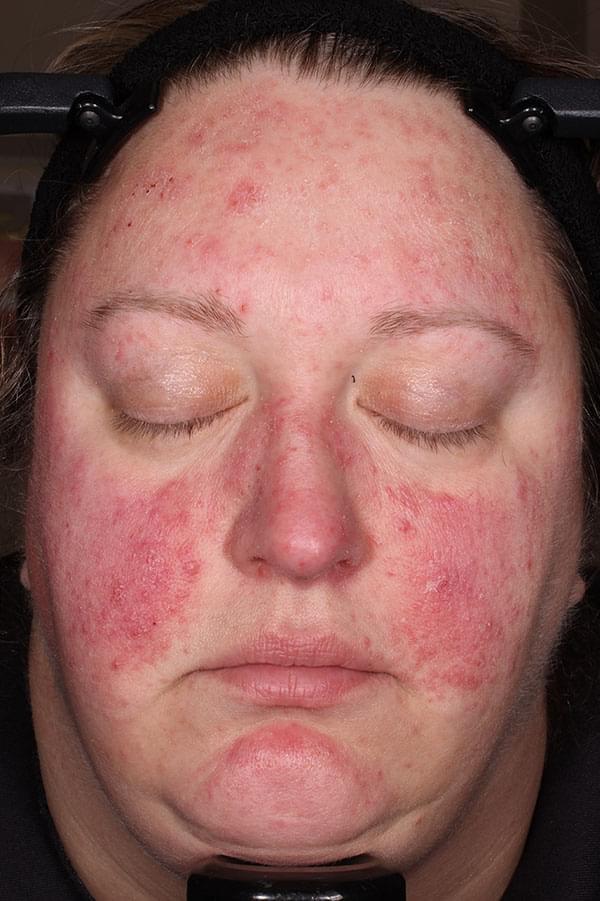
IGA SCORE=3
IGA SCORE=2
BASELINE
WEEK 12
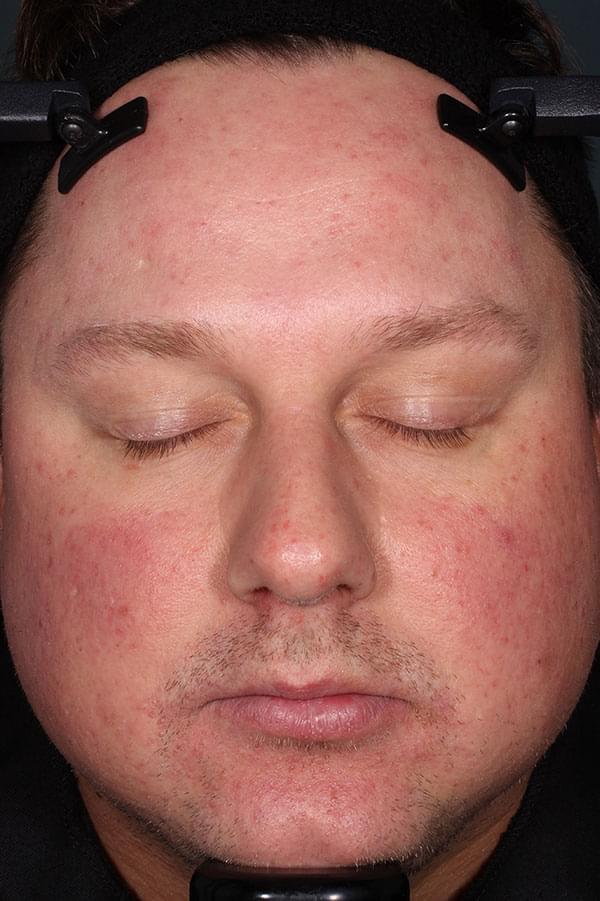
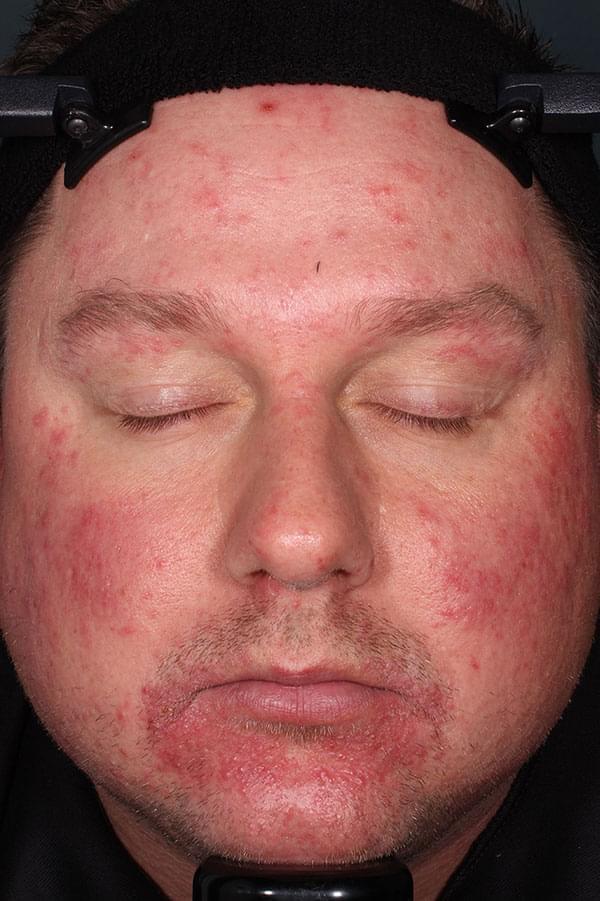
||Please see full clinical efficacy data above.
Contraindications
Persons who have shown hypersensitivity to any of the tetracyclines or any other ingredient in ZILXI.
Warnings and Precautions
Flammability: The propellant in ZILXI is flammable. Instruct the patient to avoid fire, flame, and smoking during and immediately following application.
ZILXI is a topical foam. While systemic absorption of ZILXI is low, and serious adverse reactions were not seen in clinical studies, the following adverse reactions associated with oral minocycline should be considered:
Adverse Reactions
The most common adverse reaction reported during clinical trials of ZILXI was diarrhea.
Indications and Usage
ZILXI (minocycline) topical foam, 1.5% is a tetracycline-class drug indicated for the treatment of inflammatory lesions of rosacea in adults.
Limitations of Use: This formulation of minocycline has not been evaluated in the treatment of infections. To reduce the development of drug-resistant bacteria as well as to maintain the effectiveness of other antibacterial drugs, ZILXI should be used only as indicated.
Please click to access full Prescribing Information.

Contraindications
Persons who have shown hypersensitivity to any of the tetracyclines or any other ingredient in ZILXI.
Warnings and Precautions
Flammability: The propellant in ZILXI is flammable. Instruct the patient to avoid fire, flame, and smoking during and immediately following application.
ZILXI is a topical foam. While systemic absorption of ZILXI is low, and serious adverse reactions were not seen in clinical studies, the following adverse reactions associated with oral minocycline should be considered:
Adverse Reactions
The most common adverse reaction reported during clinical trials of ZILXI was diarrhea.
Indications and Usage
ZILXI (minocycline) topical foam, 1.5% is a tetracycline-class drug indicated for the treatment of inflammatory lesions of rosacea in adults.
Limitations of Use: This formulation of minocycline has not been evaluated in the treatment of infections. To reduce the development of drug-resistant bacteria as well as to maintain the effectiveness of other antibacterial drugs, ZILXI should be used only as indicated.
Please click to access full Prescribing Information.
REFERENCES